Aqua Medic reactor 1000
€90,00 Original price was: €90,00.€85,00Current price is: €85,00.
Aqua Medic reactor 1000 is a Closed CO2-reactor for external use for aquaria up to 2,000 litres
In stock
Aqua Medic reactor 1000 is a Closed CO2-reactor for external use for aquaria up to 2,000 litres
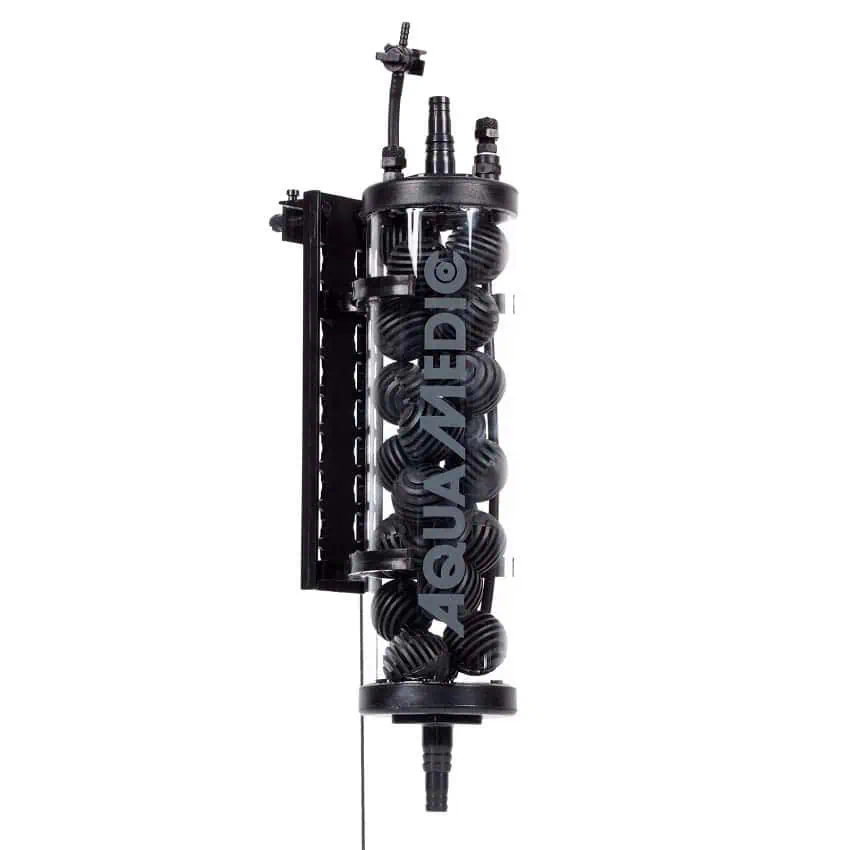
The Reactor 1000 is a hermetically sealed unit designed for efficiently introducing CO2 into larger aquariums. This powerful system can support aquaria up to 2,000 litres (approximately 500 gallons), making it an ideal solution for large-scale setups.
The unit comes with a universal mounting plate, allowing for flexible installation. You can mount it either outside the aquarium or inside the cabinet, depending on your space and setup preferences. For ease of use, it connects to a 16 mm hose.
The recommended pump power ranges from 1,500 to 2,500 l/h (approximately 396 to 660 gallons per hour), ensuring effective CO2 distribution in your aquarium.
Technical Data
- Water connection: 12/16 mm
- CO2 connection: 6/4 mm
- Water flow: min. 1,000 – max. 2,500 l/h
- Total height: 37 cm; Diameter: 80 mm
- Setup: Internal or external
- NO hoses are included
Increasing the Carbonate Hardness in Aquariums
The carbonate hardness (KH) in aquarium water, whether freshwater or saltwater, should be maintained at a minimum of 4-6 KH. When the KH level falls below this threshold, it becomes difficult to stabilize the pH-value. Biological processes, such as germ reactions, naturally produce acids that reduce carbonate hardness over time.
Other factors that consume carbonate hardness include filtration over peat or the introduction of strong acids. If you are using peat filtration, it is crucial to perform a weekly check of the carbonate hardness. If the level drops below 4 KH in freshwater, it is important to raise the KH level. We recommend using aqua+KH tablets from Aqua Medic for this purpose.
Maintenance and Care for Carbonate Hardness
The amount of dissolved CO2 in the water depends largely on the carbonate hardness. Higher KH allows more CO2 to dissolve at the same pH level. The limit of harmful CO2 concentration also depends on the carbonate hardness.
To maintain balanced water chemistry, free carbonic acid (dissolved CO2 gas) is required. This is essential for keeping calcium and magnesium ions dissolved, which are responsible for building carbonate hardness. Free carbonic acid, also known as equivalent carbonic acid, is particularly important for plants during photosynthesis. The ideal balance between carbonate hardness and CO2 is achieved at a pH of 7.1-7.4 in freshwater and 8.1-8.4 in saltwater.
When this balance is maintained, the amount of CO2 is safe for fish and does not depend on the KH level. Plants use equivalent carbonic acid during photosynthesis, and if it is not continuously replenished, the carbonate hardness can decrease, leading to biogenetic decalcification, which should be avoided.
It is essential to replace only the CO2 consumed by plants and not to oversupply CO2 to the aquarium. For a healthy environment, achieving the right balance of calcium and carbonic acid is critical. Only when this balance is maintained can plants receive adequate CO2 for growth.
| Weight | 2 kg |
|---|